Selective Discharge and the purification of Copper
The rule governing which ion would be discharged is the reactivity series. This is because the more reactive an atom is, the less electronegative it is. As such, it has a lower tendency to gain electrons. In the case of Sodium and Hydrogen ions, since sodium ions are more reactive than hydrogen ions, the hydrogen ions are discharged instead of the sodium ions. Thus we say hydrogen ions are reduced to form hydrogen atoms.
Equation: H+ + e- ---> H
Hydrogen atoms join to form diatomic molecule:
H + H ---> H2
H + H ---> H2
Test for hydrogen gas : Insert a lighted splint above aqueous Sodium Chloride solution near the cathode, lighted splint extinguishes with a "pop" sound.
Both Chloride and hydroxide ions are attracted to the positively charged anode. However, only the chloride ions are discharged, to produce chlorine atoms, Thus, we say chloride ions are oxidised to form chlorine atoms.
Equation: Cl- ---> Cl + e-
Chlorine atoms join to form chlorine gas:
Cl + Cl ---> Cl2
Cl + Cl ---> Cl2
Test for Chlorine gas: Chlorine gas turns damp blue litmus paper red then bleaches it.
In the case of anions, halide ions like chloride, bromide and iodide will be discharged. Sulfate ions are not discharged, hydroxide ions are discharged. However, this only applies to concentrated solutions.
4OH- (aq) --> O2(g) + 2H2O (l) + 4e-
Test for Oxygen gas: Insert a glowing splint above solution near the anode end. If splint rekindles, oxygen gas is present.
In dilute solutions, for instance when the concentration is 1.0mol/dm^3, or less, hydroxide ions are discharged except in the cases of copper and silver. This is also known as the concentration effect, another factor affecting the selective discharging of ions during electrolysis. However, concentration only affects the anions and not the cations.
The kind of electrodes used also affect the selective discharge of ions. In a usual electrolytic cell, inert electrodes like carbon or graphite would be used. However, in other instances, like the electrolysis of Copper (II) Sulfate solution, Copper is used as the electrode.
In Copper(II) Sulfate solution the ions present are Copper(ii) cation, Sulfate anion, Hydrogen cation and Hydroxide anion.
If we are using inert electrodes, like carbon, Copper(ii) and hydroxide will be discharged to give Copper metal and Oxygen gas. Hydrogen cation and Sulfate will be left behind, so the pH of the solution decreases and becomes more acidic as electrolysis progresses, and the intensity of the blue color fades.
If however, copper strips are used as the electrodes, instead of the Copper(II) ions in the solution being discharged, the copper strip at the anode begins eroding and the Copper metal is oxidised to form Copper(II) ions.a
Meanwhile, Copper(II) ions will be travelling to the copper cathode to become Copper metal again. Hence a pink deposit at the anode is observed as newly formed copper metal has a pink appearance. Thus we say copper(II) ions are reduced to form copper metal.
The blue copper sulfate solution will remain blue, since the concentration of Copper(II) ions in the solution doesn’t change. Any increase from the erosion of the anode is balanced by the discharge of ions at the cathode.
Therefore, another factor influencing selective discharge of ions is the type of
electrodes used in the electrolytic cell !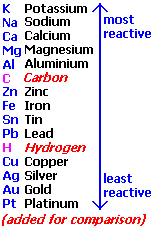

No comments:
Post a Comment